Hydrogen and Stability in Oxide Semiconductors
J. Robertson, Y. Guo,
Engineering Dept, Cambridge University, Cambridge CB2 1PZ, Uk
E-mail:
Amorphous oxide semiconductors (AOS) are of great interest for use in higher mobility
thin film transistors for displays, for example as OLED drviers. They have a higher mobility
than hydrogenated amorphous silicon (a-Si:H). In a-Si:H prime purpose of hydrogen is to
passivate the dangling bond (DB) defects, forming Si-H bonds. The resulting Si-H bonding
states lie deep in the Si valence band. In AOS’s, the main focus has been on carrier mobility,
the bonding is ionic, with the metal s states forming the conduction band, and the O p states
forming the valence band. There is an instability from light induced bias stress (NBIS). The
exact mechanism is still debated, between O vacancy [1], O interstitial [2,3], or some
hydrogen related process [4,5].
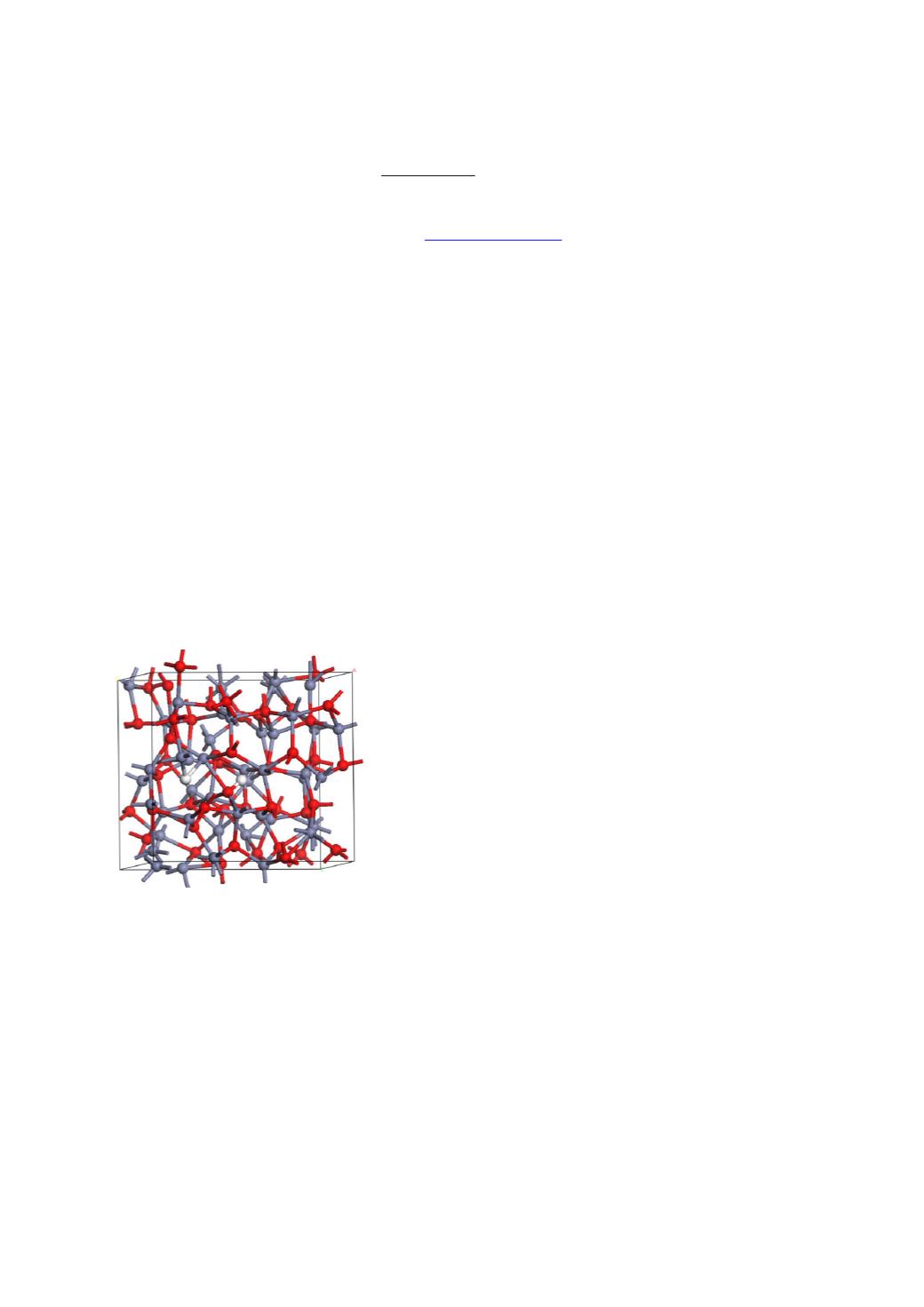
Here, we consider ZnO as the canonical example. Hydrogen can be introduced into the ZnO
network, where it can bond to the O site or the Zn site, see Fig. 1. It can also bond within an
O vacancy. IN principle, hydrogen’s ability to bond to both O and Zn sites, allows it to
passivate both O and Zn DBs. But, unlike in a-Si:H, the Zn-H bonding states lie at the bottom
of the band gap. This they are accessible to optical excitation or to hole trapping. They lie in
the same energy range as the O interstitial states, which have also been considered as causing
instability.
Thus we describe here the bonding behavior of H in a-ZnO, and describe the properties of
H related states in AOS’s, and how these might relate to the NSIB.
Fig. 1. Amorphous ZnO network with hydrogen additions(grey = Zn, red = O, white = H)
1.
B Ryu, H K Noh, E A CHoi, K J Chang, APL 97 022108 (2010)
2.
H H Namh et al, Phys State Solidi B 249 1277 (2012)
3.
J Robertson, Y Guo, APL 104 162102 (2014)
4.
H H Nahm et al, Sci Rep 4 4124 (2014)
5.
Y Kang et al, Adv Electron Mats 1 14006 (2015)
P 10
-134-


