+2 charged oxygen vacancy: a shallow-donor native point defect in ZnO revealed by
18
O
self-diffusion in isotopic heterostructures
Lishu Liu
1
, Zengxia Mei
1
*, Aihua Tang
1
, Alexander Azarov
2
, Andrej Kuznetsov
2
, Qi-Kun Xue
3
and
Xiaolong Du
1
1
Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of
Sciences, Beijing 100190, P. R. China
2
Department of Physics, University of Oslo, Oslo P.O. Box 1048, NO-0316, Norway
3
Department of Physics, Tsinghua University, Beijing 100084, China
Contact e-mail
As a third-generation semiconductor, ZnO has been gaining momentum since the first
experimental demonstration of optically pumped lasing in ZnO at room temperature in 1997.
Progress towards ZnO-based optoelectronic devices and applications, however, has been
impeded largely by the unintentional and seemingly unavoidable n-type conductivity of ZnO
materials that makes stable p-type doping extremely daunting. In order to solve this most
puzzling problem in ZnO for more than 50 years, it is highly desirable to understand the
origin of the unintentional n-type conductivity.
Here, by conceiving and growing oxygen-isotope ZnO heterostructures with
delicately-controlled chemical potential and Fermi level, we investigate self-diffusion process
of oxygen atoms in ZnO, and demonstrate unambiguously that, in contrast to the general
belief of their neutral attribute, the oxygen vacancies in ZnO are actually +2 charged, even
near the conduction band minimum. We further demonstrate that oxygen vacancies are the
dominant donor-like native point defects and thus responsible for the unintentional n-type
conductivity as well as the non-stoichiometry of ZnO, which have generally been observed in
experiments. The methodology we have developed can be used to study oxygen-related point
defects and their energetics in other technologically important metal oxides such as TiO
2
and
In
2
O
3
, and thus is of general interest in oxide and semiconductor physics community.
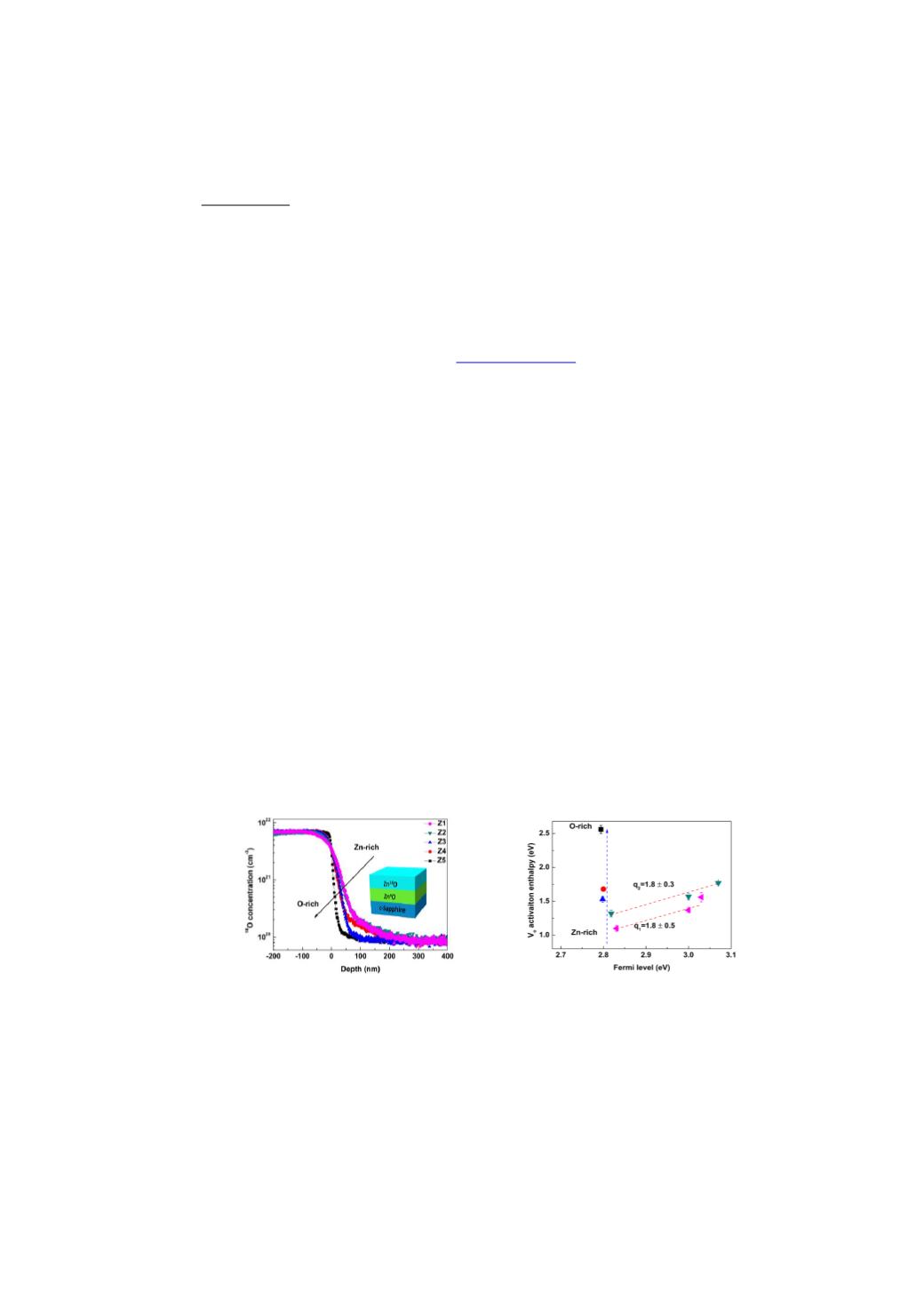
Figure (a)
18
O concentration depth profiles in as-grown samples. Samples labeled as Z1 to Z5 were grown with
decreased Zn/O ratio. The inset shows the schematic structure of these samples.
(b) Activation enthalpy of V
O
as a function of Fermi level. Dashed lines illustrate an ascending trend for
△
H
a
with increased Fermi level for two batches of samples: Z1 to Z1-2 and Z2 to Z2-2. As shown
by the red dashed lines, the charged state of V
O
is obtained by fitting the two batches of samples
using linear relation, respectively.
△
H
a
as a function of chemical potential is also included for
Z1-Z5 samples, as illustrated by the blue dashed lines.
(b)
(a)
I 23
-132-


